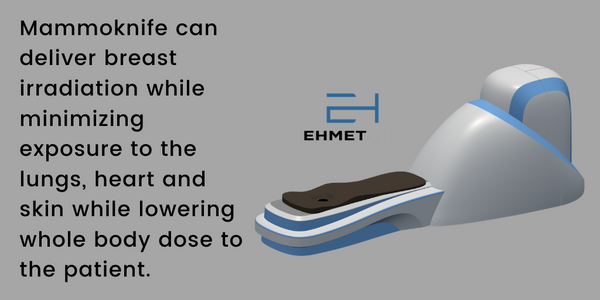
Ehmet Health is a privately held, emerging medical device company with an innovative device for providing radiation as a treatment for breast cancer. Radiation therapy remains a mainstay of treatment for breast cancer patients, yet hospitals and medical centers face significant challenges in providing all patients safe and cost-effective care. Moreover, meaningful logistical impediments keep many patients in rural areas of the country from receiving treatment. Ehmet’s MammoKnife was designed to address many of these challenges. The product targets a multi-billion dollar market opportunity with a rapid path to clinical use in the U.S. and Europe.
Despite vast improvements in early detection and screening, breast cancer remains the leading cancer affecting women in the United States. According to data from Cancer.org, there were over 268,000 new cases of breast cancer in 2018, over 99% of which were women. This represented 30% of all new cancer cases for women. Data from the National Cancer Institute (NCI) between 2014 and 2016 show one-in-eight women will be diagnosed with breast cancer at some point during their lifetime; and although 5-year survival rates are approximately 90%, breast cancer still results in over 41,000 deaths per year in the U.S.
Treatment for breast cancer usually involves either breast-conserving surgery (BCS) lumpectomy (surgical removal of the tumor and surrounding tissue) or mastectomy (surgical removal of the entire breast), depending on tumor characteristics (e.g., size, hormone receptor status, and extent of spread) and patient preference. Radiation to the breast is recommended for most patients having a lumpectomy and even some following mastectomy. In fact, about 50% of all breast cancer patients will have radiation at some point during their treatment.
The Problem with Current Radiation Protocols
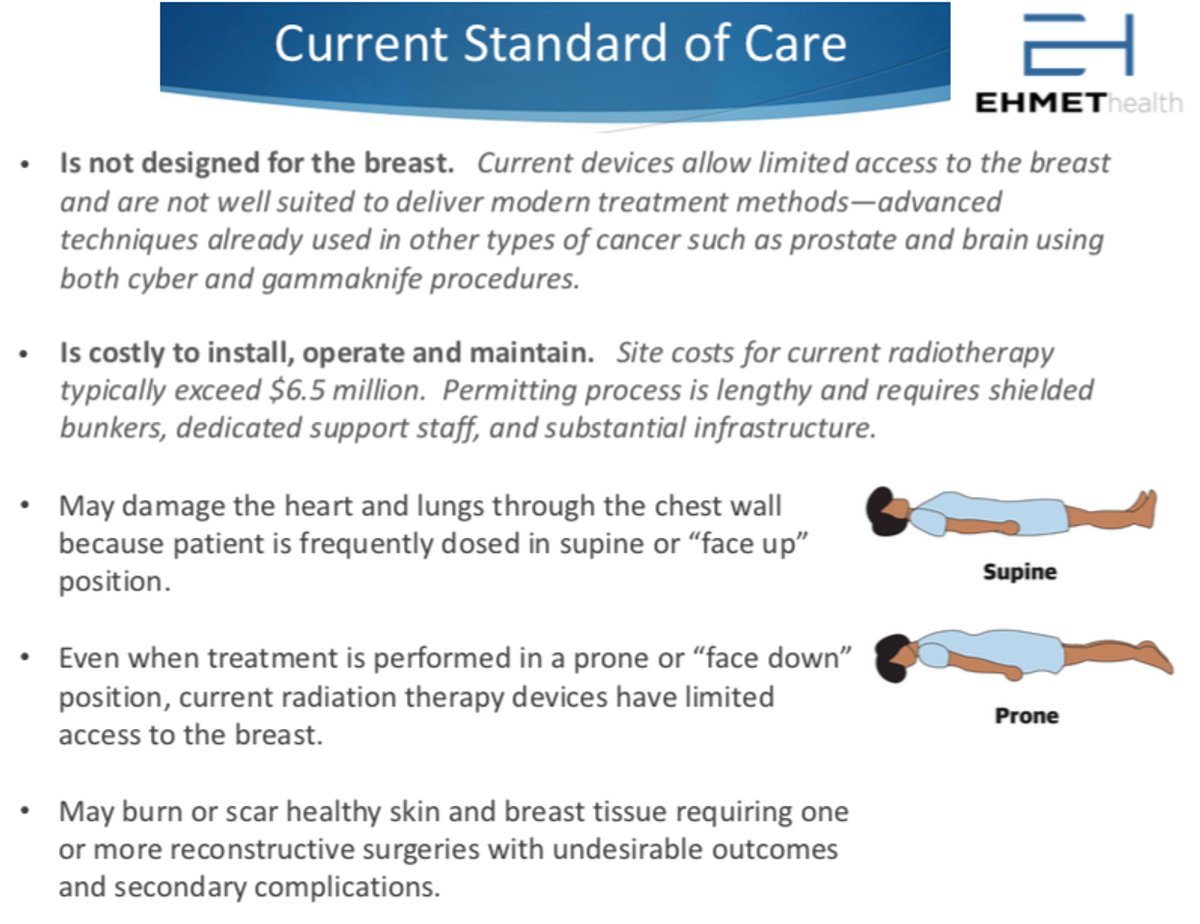
Despite generally good outcomes, there are still short-term and long-term side effects with radiation. Short-term effects including pain, burning, and skin damage. Long-term effects include structural damage to the skin and breast, scarring, and discoloration, as well as lymphedema and potential damage to the nearby heart or lungs. For many women, the fear of these side effects creates significant anxiety prior to treatment.
One of the reasons why radiation results in side effects is that current radiation machines are outdated or not specifically designed for breast cancer. Breast cancer typically accounts for more than one-quarter of a radiation department’s typical workload, hospitals usually purchase expensive general radiation machines (linear accelerators) that can be used to treat other cancers, such as brain, head and neck, or even prostate cancer. The architecture of these machines is not optimum for treating breast cancer. Breast radiation in the supine position typically uses beams from only two angles, which can result in excessive burning or scarring on the skin. In addition, supine treatment is associated with damage to critical organs such as the heart and lungs as well as undesirable radiation to other healthy tissue. In fact, the American Heart Association (AHA) began warning patients and physicians about this risk in 2018. While radiation therapy in the prone position mitigates some issues, setup time is longer and unimpeded treatment beam access to the breast is limited.
Radiation departments, or “bunkers”, are also expensive to build and maintain. It might cost between $5 and $7 million to build a bunker and another $5 million to equip the facility. This means that over 90% of cancer radiation is done at major cancer centers in large cities or at academic hospitals also found near large cities. These bunkers are just too expensive for most rural hospitals to build and outfit. As a result, there are significant logistical and financial challenges to rural cancer care in the U.S. because not all cancer patients live near these major centers.
A study in Iowa found that 50% of patients travel more than 30 minutes to receive treatment, and some patients in most rural areas end up traveling three-times as long. With some treatments taking place daily or several times a week, this creates significant emotional stress for patients.
Travel also creates financial hardship because beyond simply paying for gas and travel expenses, patients need time off from work to receive treatment. The distance also makes it difficult for rural cancer patients to participate in clinical trials where they might receive a step-up in care or financial support from the sponsor. This is the main reason why survival rates and outcomes are worse in low-income areas of the U.S., and abysmally low in under-developed regions outside the U.S.
A New Direction for Breast Cancer Radiation
One solution to many of the above challenges is to bring the treatment closer to the patient. A machine that is self-shielding can be used outside of a traditional radiation bunker. This could save a rural hospital millions of dollars and make it so that more patients have access to treatment. If that machine is specifically built for breast cancer applications, advanced treatment plans can be used that result in less damage on the skin on the outside and patients can be positioned properly to minimize systemic spill-over to nearby organs on the inside. Clinical data show that accelerated partial breast irradiation is non-inferior to whole breast irradiation in terms of long-term outcomes, but many women still choose whole breast irradiation because it results in less cosmetic damage and the eventual need for reconstructive surgery.
Ehmet’s MammoKnife as a Solution
MammoKnife is the first self-shielded linear accelerator designed specifically to treat breast cancer patients. MammoKnife has many of the key attributes discussed above that not only increase access to care but are designed to improve outcomes as well.

- MammoKnife is Self-Shielded, which means that use does not need to be restricted to a radiation bunker. In fact, MammoKnife has been designed for deployment on a standard trailer (view 3D animation) allowing for a first of its kind platform delivering precision therapy in a mobile setting. In the future, Ehmet plans to deliver a turn-key system that can be deployed to any urban or rural location by partnering with community medicine and outpatient centers. This solves the issue of Economics for rural hospitals and should work to significantly increase access to care for all patients.
- MammoKnife leverages the Current Standards. The 6-MV linear accelerator, which is the standard for whole or partial breast irradiation, can be used for stereotactic surgery, compatible with most treatment planning systems and uses existing CPT codes for reimbursement. The device also integrates target imaging to reduce the need for a separate CT simulator and allow for adaptive planning reimbursement and targeted treatment.
- Patients are treated in the Prone Position, which offers superior treatment outcomes and reduces the risk of secondary dose to nearby organs such as the lungs or heart. The device rotates the patient 360 degrees (view 3D animation), which optimizes target intensity and further minimizes the off-target effects on healthy tissue.
- As reimbursement in the U.S. moves to the Alternative Payment Model (APM), much as it is in almost all areas outside the U.S., hospital administrators are going to have to figure out how to provide a cost-effective solution while delivering the same high-quality care to patients. MammoKnife addresses the issue of value-based care and payment bundling by delivering efficacious levels of treatment in a far more efficient protocol.
A Significant Market Opportunity
With 268,000 new cases of breast cancer in the U.S. each year and another 400,000 in Europe, the breast cancer radiation market represents an enormous market opportunity. Ehmet has an opportunity to not only sell the MammoKnife device to large medical centers in urban areas looking to expand their breast cancer radiation business, but also tackle the wide-open rural market through mobile platforms and turn-key solutions. At approximately $2.5 million per unit, with only 10% market penetration in the U.S. and Europe, MammoKnife is a potential $4.0 billion product. Ehmet also has the opportunity for annuity-like revenues from service contracts, which will likely cost 8-10% of the unit sales price and run for a decade after placement.
Ehmet is currently working with leading breast cancer specialists to identify the best practice methods and techniques required to achieve that 10% penetration goal. The mobile setting has the potential to be a real game-changer because it offers large academic centers and community hospitals an opportunity to expand their brand beyond the city in which they are located. It’s a particularly attractive opportunity for emerging markets.
MammoKnife is being developed under the FDA’s 510(k) regulatory pathway because the device has “substantial equivalence” to existing machines. This is an area of expertise for the current management team. The U.S. 510(k) pathway does not require clinical trials prior to approval. Moreover, reimbursement codes in the U.S. for the therapies delivered by the machine are already in place. This greatly simplifies the path to market and removes significant risk and cost of commercialization. The pathway in Europe is similar, and that presents a tremendous opportunity for Ehmet and MammoKnife in the coming decade.